AI and FDA
Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices
October 19, 2023 update: 171 Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices were added to the list below. Of those newly added to the list, 155 are devices with final decision dates between August 1, 2022, and July 30, 2023, and 16 are devices from prior periods identified through a refinement of methods used to generate this list.
This list contains publicly available information on AI/ML-enabled devices. The FDA assembled this list by searching FDA’s publicly-facing information, as well as by reviewing information in the publicly available resources cited below (*) and in other publicly available materials published by the specific manufacturers.
Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices: Oct 2023 Update
2023-12-12: The Current State Of Almost 700 FDA-Approved, AI-Based Medical Devices
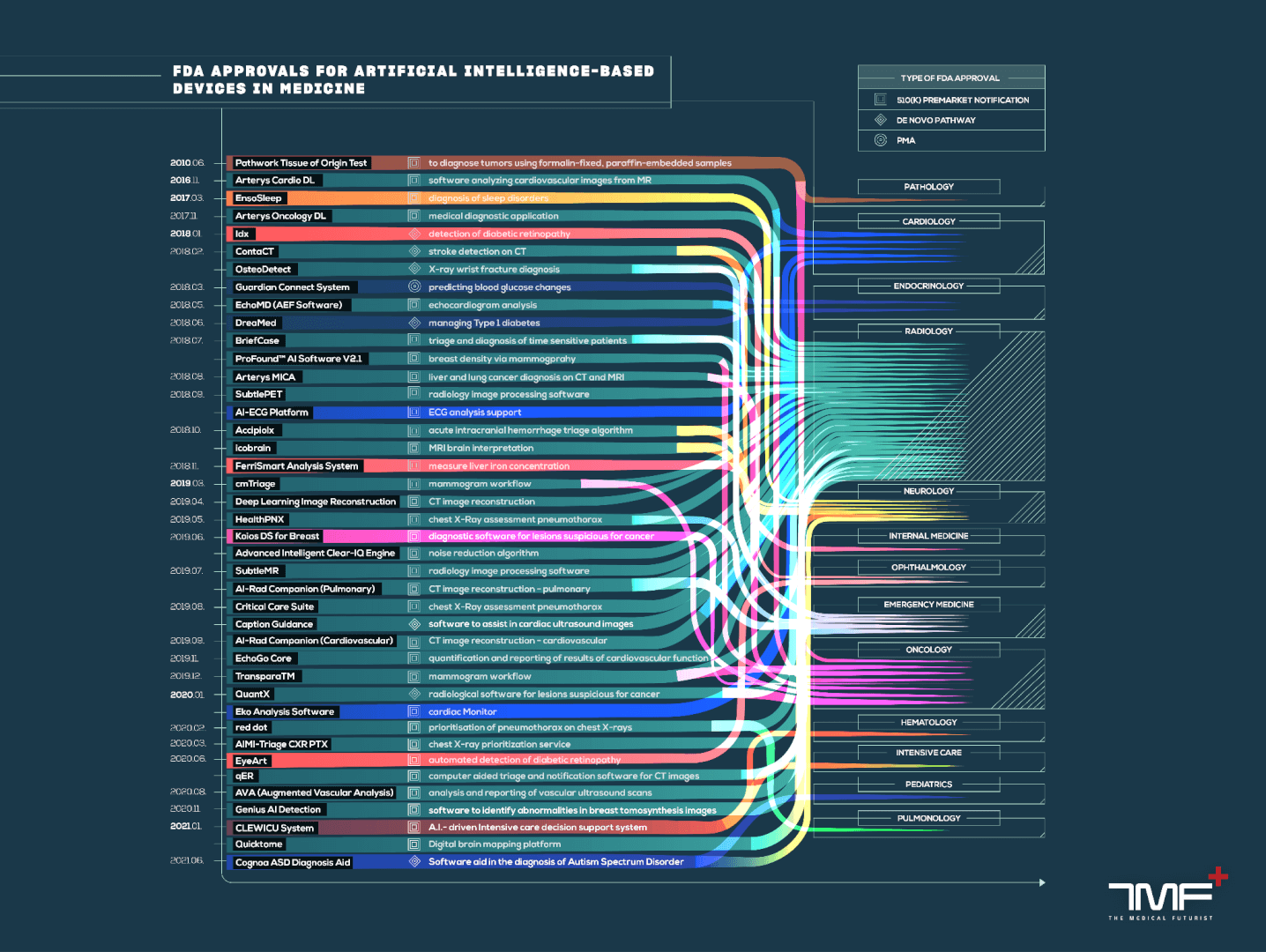
AI Use Cases
The US Government HHS lists Department of Health and Human Services: Artificial Intelligence Use Cases Inventory): “an inventory of HHS AI use cases that not only satisfies the executive order requirements, but also increases awareness of and cross-agency collaboration on AI initiatives.”
Includes a CSV file.